Formulation
NASALER Gelatin softgel capsule (size 12 oval) : gelatin soft capsule of 1058 mg containing a quantity of turmeric extract corresponding to 42.0 mg of curcumin, 65.0 mg of natural quercetin from Sophora japonica L. (pagoda tree or Chinese scholar tree) and and 2.25 µg of vitamin D3 (cholecalciferol).
Clinical Data
NASALER and Allergic Rhinitis
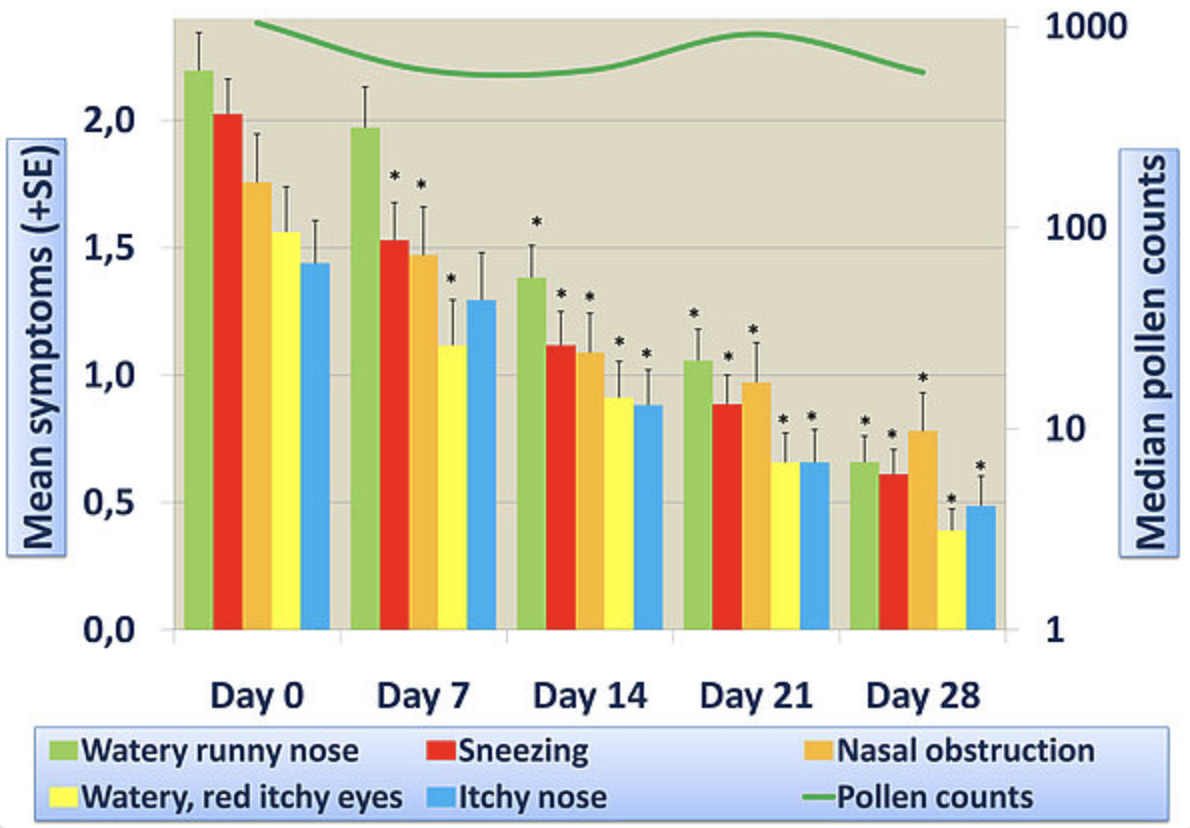
Through its unique formulation techniques, bioXtract has managed to create with NASALER a product with significantly higher bioavailability than the individual pure extracts, as proven by our in vivo trials.
These active principals act on the symptoms of allergic rhinitis, caused by inhaled substances (for example pollen, dust mites, mould spores) to relieve:
- itching, sneezing and runny eyes and nose - through the properties of natural antihistamine quercetin,
- nasal congestion, and the underlying inflammation in the nasal membranes, through the broad spectrum anti-inflammatory effects of curcumin (in an improved bioavailable formula).
At this level of availability, the quercetin and curcumin in NASALER will together have an effect on both the immediate symptoms of allergic rhinitis and the later stage nasal congestion.

NASALER is delivered with a complete package of information, including bioXtract proprietary:
- Pre-clinical efficacy trial evaluating the action of active principals on several models of allergic inflammation in the nasal membrane,
- Pre-clinical acute oral toxicity trial in rats showing no particular effect and classifying the product in the danger 5 category DL50 superior 5,000 mg/kg,
- Phase I clinical trial, assessing the bioavailability of NASALER's curcumin,
- Open-label clinical trial data showing efficacy of NASALER on the symptoms of allergic rhinitis.
See slides hereunder for more information.