Formulation
RIFENCIN Gelatin softgel capsule (size 10 oval) : gelatin soft capsule of 920 mg containing a quantity of turmeric extract corresponding to 42.0 mg of curcumin in highly bioactive form and 25 mg of sweet fennel essential oil.
Clinical Data
RIFENCIN and GI Disease
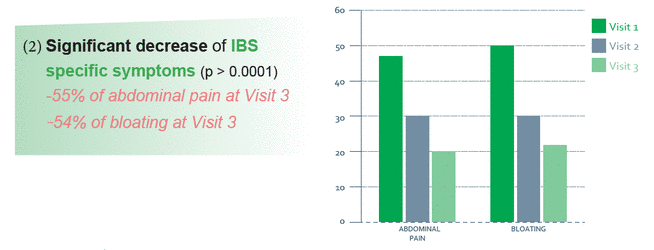
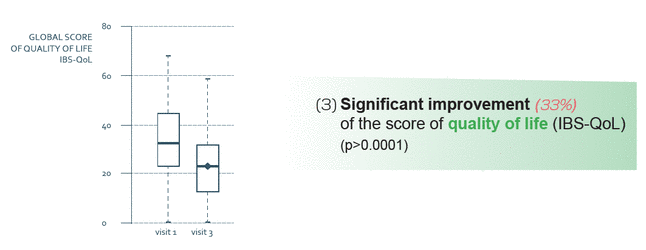
RIFENCIN is a natural remedy whose active principals act on the symptoms of gastro-intestinal disorders like Irritable Bowel Disease (IBD) and Irritable Bowel Syndrome (IBS). The combination of curcumin and fennel offers a combination of complementary active principals.
Curcumin is known in traditional medicine for its capacity to increase bile production, to help relieve the symptoms of indigestion :
- feeling of "full stomach"
- flatulence
- slow digestion
Fennel is also traditionally known for the symptomatic treatment of :
- mild gastrointestinal spastic disorders
- flatulence
- bloating

RIFENCIN is delivered with a complete package of information, including bioXtract proprietary:
- Pre-clinical acute oral toxicity trial in rats showing no particular effect and classifying the product in the danger 5 category DL50 superior 5,000 mg/kg,
- Phase I clinical trial demonstrating bioavailability of RIFENCIN's active principals. (Complete report available on request),
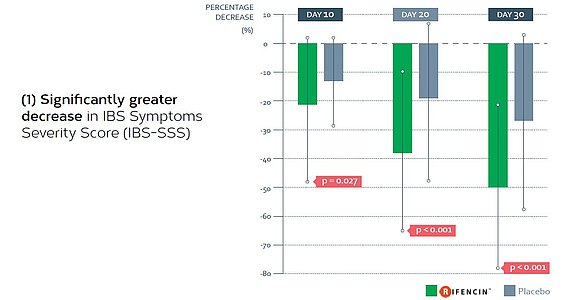
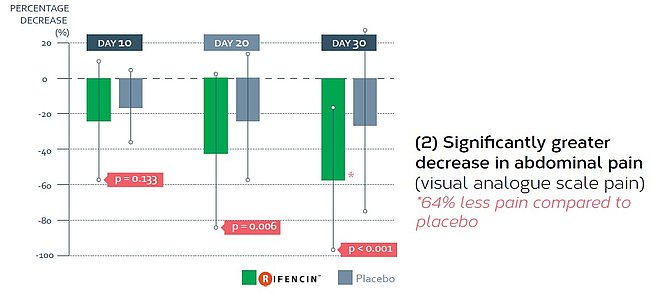
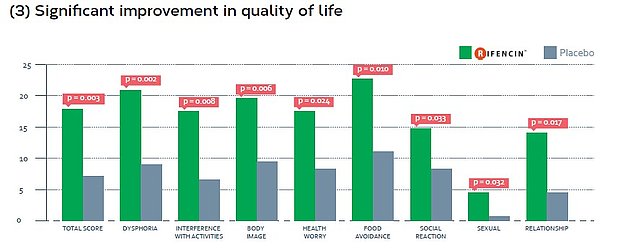
- Phase II randomised, double-blind, multicentre, placebo controlled clinical trial evaluating the efficacy of RIFENCIN in 120 patients suffering from functional gastrointestinal symptoms on a period of 30 days,
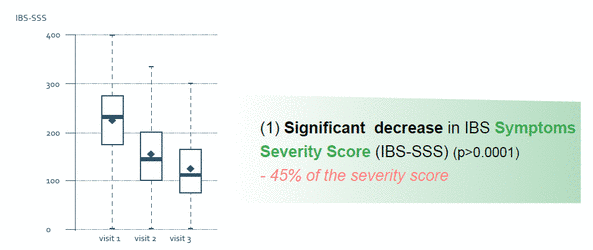
- Observational clinical study, prospective, not-randomized, real-life of RIFENCIN on 253 patients suffering from Intestinal Bowel Syndrome (IBS)
See slides hereunder for more information.